🚀 12 RPA scenarios for Pharmacovigilance.
12 use cases for Robot Programming Automation in Pharmacovigilance that simplify the job-to-be done of your PV team. In perfect automation.
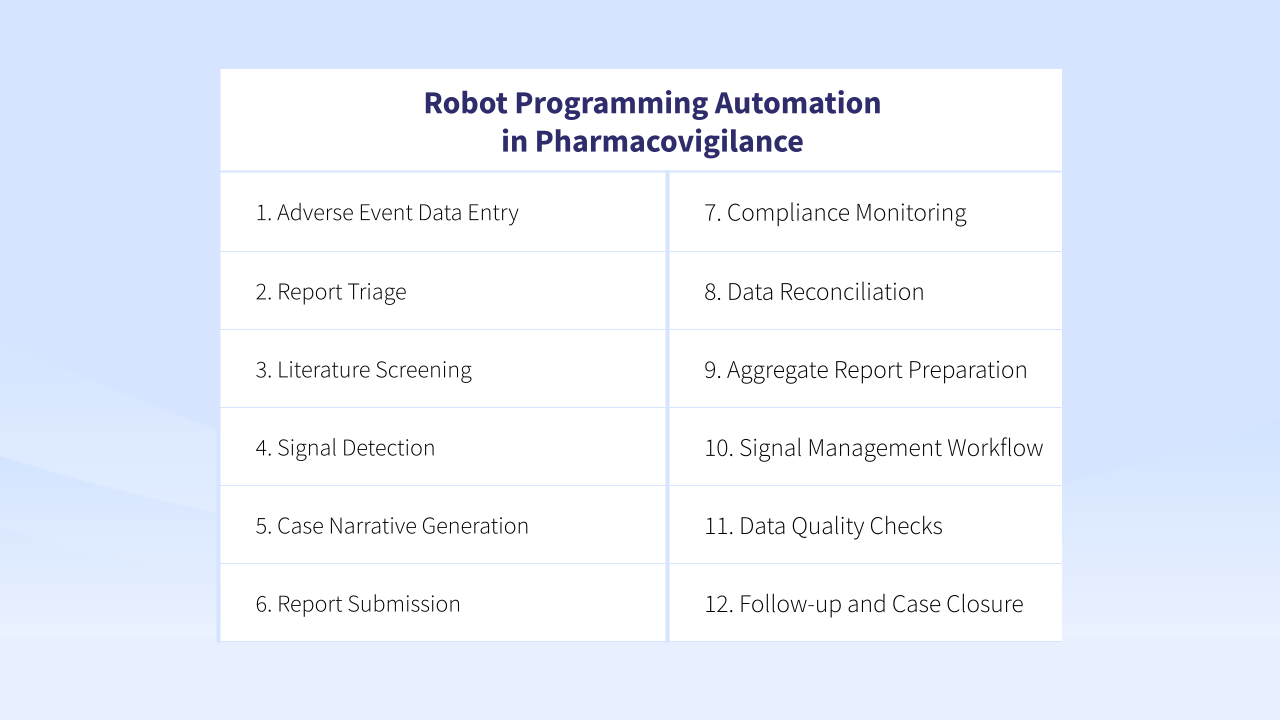
We have gathered the most common questions of pharmacovigilance specialists and grouped their job-to-be done in 12 scenarios that can be addressed with RPA (Robot Programming Automation).
As medical data is becoming more and more digitized, and it is increasing at faster rates, these tasks are most likely going to become a key aspect in your PV monitoring.
#1 Adverse Event Data Entry 📧
A pharmaceutical company receives a substantial number of adverse event reports daily from various sources, including healthcare professionals, patients, and clinical trial investigators. These reports arrive through different channels, such as emails, and online forms. The manual data entry process is time-consuming, prone to errors, and can lead to delays in processing crucial safety information.
✅ You can setup a bot to read and monitor a specific inbox where AE are collected, processed, transformed into an item that then gets ingested into a database. This will ensure your database getting populated from data entries such as emails.
#2 Report Triage ⛔
RPA bots can be trained to classify adverse event reports based on their severity, potential causality, or other criteria. This automated triage can help prioritize the review process, ensuring that critical cases are addressed promptly.
➡️ For example by downloading FAERS data from FDA and extracting adverse effect and level of danger, you can easily build a classifier.
#3 Literature Screening 🧾
RPA can be employed to automate the screening of scientific literature for relevant safety information. The bots can scan through medical journals, articles, and other publications, extracting data related to adverse events associated with specific drugs or classes of drugs.
✅ A quick example of that is PapersHive Monitoring.
#4 Signal Detection 💊
RPA can assist in signal detection by analyzing pharmacovigilance databases and identifying potential safety concerns or emerging patterns that require further investigation.
- Co-occurrence of drug (or ingredient) with side effect.
- Co-occurrence of one side effect with another side effect.
- Getting thresholds and understanding when a side effects is recognized as such.
➡️ Data Analysis on your safety reports and AEs is a goldmine of information.
#5 Case Narrative Generation 🧐
RPA bots can generate case narratives by extracting relevant information from the database and creating structured reports that comply with regulatory requirements.
✅ For example Clinical Trials are quite structured when it comes to inclusion criteria: age, sex, gender, weight, ethnicity, etc. From this, a voluntary MedWatch report could be easily generated.
#6 Report Submission 🧑🎓
RPA can automate the process of compiling and submitting safety reports to regulatory authorities, ensuring timely and accurate submissions.
- Instead of re-creating, you can download and auto-fill the parameters within a report.
- This approach can also take care of reminding of an approaching deadline.
- You can even connect it to point #5 and have an automated pipeline for generating, filling and submitting reports.
#7 Compliance Monitoring ⚕️
RPA bots can be programmed to monitor compliance with pharmacovigilance regulations and internal standard operating procedures, flagging any deviations for review and corrective action.
✅ Missing a required field in a report, not having enough evidence (quantity standpoint), or not having an approval from your internal RA specialist could easily be automated with a few lines script.
#8 Data Reconciliation ♻️
RPA can assist in reconciling safety data between different systems, ensuring data consistency and accuracy.
➡️ A clinical trial in U.S. register (such as ClinicalTrials.gov) might appear also in Clinical Trials EU register. These should be addressed as one, and not two clinical trials. Similar for trials appearing on PubMed, and patents appearing on PubChem.
#9 Aggregate Report Preparation 🧑💻
RPA bots can help compile data for Periodic Safety Reports (PSURs, PBRERs, etc.) by extracting relevant information from databases and formatting it into the required templates.
✅ Extracting information from Clinical Trials into XML and ingesting it into your Safety Database, and extracting information from your Safety Database into a report valid for FDA and EMA.
#10 Signal Management Workflow
RPA can automate the workflow for signal management, including the review and assessment of potential safety signals identified during the pharmacovigilance process.
➡️ It could gather information from your Safety Database, your recent literature review and highlight co-occurrences, similarities and discrepancies.
#11 Data Quality Checks 🔍
RPA bots can be utilized to perform routine data quality checks to identify and rectify data inconsistencies or errors.
✅ The most evident on this is a specific Clinical Trial moving of phase, or a PubMed article going from pre-print to peer reviewed.
#12 Follow-up and Case Closure 🔒
RPA can be deployed to track the progress of adverse event cases, send follow-up queries to reporters, and facilitate case closure when appropriate.
- "What is the status of AE case?"
- "Did we reply regarding the AE?"
- "Can we reply now or do we need more information?"
➡️ Ever heard of these ones? The most common questions when talking to reporters. By keeping an eye with RPA on these tasks, these kind of questions won't consume more than few seconds of your time.
Conclusions 🚀
✅ RPA in Pharmacovigilance has infinite possibilities and use cases. From extracting information into a safety database, from filing and autocompleting a report to a full FDA and EMA submission.
➡️ The most important take-home lesson is that pharmacovigilance is a dynamic profession that requires critical thinking. Your RPA process should mimic this, by having your pharmacovigilance specialists assessing your outcomes.
🔥 psst: PapersHive is working towards automating 12 above goals with RPA!

Everything starts with search.
With a smart suite of search tools to help you find the information you need, when you need it. Enhance your Search Experience with PapersHive Today!
Contact Us